MAPLE Fabricated Fe³O4@Cinnamomum verum Antimicrobial Surfaces for Improved Gastrostomy Tubes
Ion Anghel, Alina Georgiana Anghel: ENT, “Carol Davila” University of Medicine and Pharmacy, Traian Vuia no.6, Bucharest 020956,
Romania; E-Mails: dr_alina.anghel@yahoo.com (A.G.A.); ionangheldoc@yahoo.com (I.A.)
Doctor Anghel Medical Center, Theodor Sperantia Street, Bucharest 30932, Romania
Alexandru Mihai Grumezescu, Valentina Grumezescu, Alexandra Elena Oprea, Alina Maria Holban :Department of Science and Engineering of Oxide Materials and Nanomaterials,
Faculty of Applied Chemistry and Materials Science, Politehnica University of Bucharest,
Polizu Street no 1-7, Bucharest 011061, Romania; E-Mails: grumezescu@yahoo.com (A.M.G.);
valentina_grumezescu@yahoo.com (V.G.); elena_oprea_93@yahoo.co.uk (A.E.O.);
alina_m_h@yahoo.com (A.M.H.)
Alexandru Mihai Grumezescu, Valentina Grumezescu, Alina Maria Holban :AMG Transcend, Polizu Street no 1-7, Bucharest 011061, Romania
Mariana Chirea: Departamento de Química Fisica, Universidade de Vigo, 36310 Vigo, Pontevedra, Spain
Valentina Grumezescu, Gabriel Socol: National Institute for Lasers, Plasma & Radiation Physics, Lasers Department, P.O. Box MG-36,
Bucharest-Magurele, Bucharest 769231, Romania; E-Mail: gabriel.socol@inflpr.ro
Florin Iordache: Flow Cytometry and Cell Therapy Laboratory, Institute of Cellular Biology and Pathology “Nicolae
Simionescu” (ICBP), Bucharest 050568, Romania; E-Mail: floriniordache84@yahoo.com
Alina Maria Holban: Microbiology Immunology Department, Faculty of Biology, University of Bucharest,
AleeaPortocalelor no 1-3, Bucharest 060101, Romania
* Author to whom correspondence should be addressed; E-Mail: mariana.chirea@uvigo.es or
mariana.chirea@fc.up.pt; Tel.: +34-986-81-86-17; Fax: +34-986-81-25-56.
Abstract: Cinnamomum verum-functionalized Fe3O4 nanoparticles of 9.4 nm in size were laser transferred by matrix assisted pulsed laser evaporation (MAPLE) technique onto gastrostomy tubes (G-tubes) for antibacterial activity evaluation toward Gram positive and Gram negative microbial colonization. X-ray diffraction analysis of the nanoparticle powder showed a polycrystalline magnetite structure, whereas infrared mapping confirmed the integrity of C. verum (CV) functional groups after the laser transfer. The specific topography of the deposited films involved a uniform thin coating together with several aggregates of bio-functionalized magnetite particles covering the G-tubes. C ytotoxicityassays showed an increase of the G-tube surface biocompatibility after Fe3O4@CV treatment, allowing a normal development of endothelial cells up to five days of incubation. Microbiological assays on nanoparticle-modified G-tube surfaces have proved an improvement of anti-adherent properties, significantly reducing both Gram negative and Gram positive bacteria colonization.
1. Introduction
Patients who are unable to move food from their mouth to their stomach such as patients suffering of neurological disorders (stroke, brain injury, amyotrophic lateral sclerosis, and impaired swallowing), as well as patient with trauma, cancer or other severe infections in the upper gastrointestinal or the respiratory tract need an alternative solution for nutrition intake.
The percutaneous endoscopic gastrostomy (PEG) technique or gastrostomy tube (G-tube) is a method of placing a tube into the stomach percutaneously, assisted by endoscopy and allowing nutrition intake in these debilitated patients [1]. G-tubes are becoming increasingly more common to provide nutrition to patients due to their decreased risk of aspiration, usually associated with
nasogastric tubes [2]. The most common complications associated with G-tube placement range from minor bleeding and poor comfort to wound infections, necrotizing fasciitis and colocutaneous fistula [3]. The reported rates of complications following G-tube placement vary from 16% to 70% [3].
Being in contact with patients’ normal microbiota, medical implanted prostheses are prone to be colonized by bacteria [4]. The failure of such devices relies on the ability of microorganisms to attach on their surfaces and form highly specialized communities called biofilms, which are extremely resistant to host defence mechanisms and antibiotic treatment [5]. Medical biofilms colonizing the surface of prosthetic devices are the cause of 60%–85% of total human acute or chronic infections and the most frequent cause of device surgical removal, associated with additional costs and patients’discomfort [6] The microbial species of clinical interest, often involved in biofilm associated infections belong to a very wide spectrum, from Gram positive (Staplylococcus aureus and S. epidermidis) to Gram negative pathogens (Escherichia coli and Pseudomonas aeruginosa) and to different members of the Candida genus (particularly C. albicans and C. parapsilosis) [7].
Conventional treatments for device-associated infections typically consist of long-term therapy with a combination of antibiotics, which may lead to side effects and contribute to low patient compliance. Significantly, the increasing emergence of drug resistance to commonly used antibiotics and antifungal has made critical the need for the identification of novel therapeutics and approaches [8,9].
Recent advances in nanotechnology research have demonstrated that bio-functionalized nanomaterials can be used successfully for antibacterial protection of medical devices [10–19]. This protection of medical devices is easily achieved by directly coating the medical devices with a bio active antimicrobial film [20,21]. The use of synthetic drugs for antibacterial treatment of medical
devices is always accompanied by severe side effects (i.e., high toxicity and low biodegradability, increased bacterial resistance rates) and elimination of these side effects is achievable by using natural products with proved antimicrobial activity [22–27]. Plant extracts and essential oils with complex medicinal properties represent a great choice for the development of antimicrobial therapies [28–33].
The only drawbacks of these natural products are their low stability and high volatility. The functionalization of biocompatible nanoparticles with these essential oils or plant extracts would provide the means to both increase their stability and decrease their volatility [34–36].
In this work, we present the bio-functionalization of magnetic nanoparticles with Cinnamomum verum (CV) and their use for fabrication of protective antibacterial coatings of G-tubes. C. verum contains two antioxidants: proanthocyanidins and cinnamaldehyde (a phenolic compound), the latter being a very powerful anti-inflammatory compound. The most important medicinal properties of C. verum oil are: it stimulates the immune system, it is a general anti-inflammatory oil, extremely powerful antioxidant, major broad-spectrum antibiotic, antiseptic, antifungal and antiviral. It also helps prevent stomach, colon, lung, and breast cancers. Its many uses are known from Traditional Chinese
Medicine. It is expected that the bio-functionalization of Fe3O4 nanoparticles with C. verum will lead to the formation of films with strong anti-inflammatory and antibacterial activity and few or no side effects. The fabrication of these films was achieved by matrix assisted pulsed laser evaporation (MAPLE), a technique frequently used for film deposition on substrates [37–41]. MAPLE allows a better control of the film thickness and surface morphology, enhances film/substrate adhesion, facilitates the multi-layer deposition and patterning. Furthermore, being a non-contact procedure it eliminates a major source of contamination and it can be integrated with other sterile processes. The
bacterial species under study in this work were a Gram positive one, S. aureus and a Gram negative one, E. coli, which are the most frequent etiologies for severe and persistent infections associated with G-tube implantation.
2. Results and Discussion
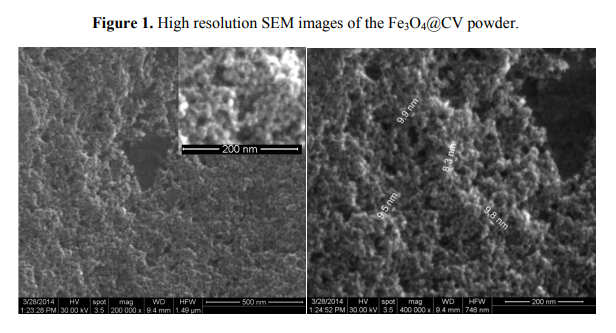
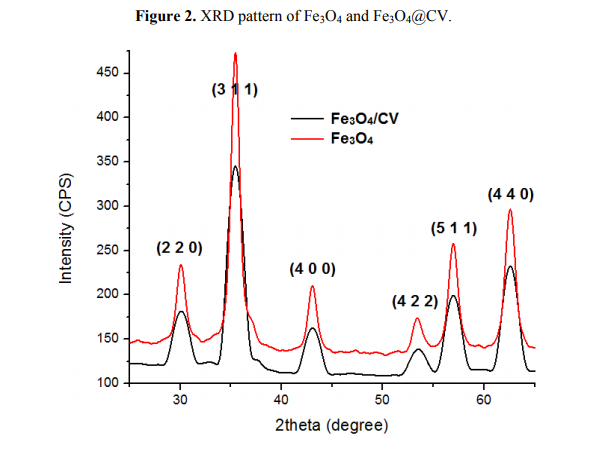
Details concerning the method used for magnetite synthesis and procedure for their deposition on G-tubes are presented in the Experimental Section. The morphology and crystallinity of the synthesized magnetite nanoparticles was analysed by TEM, SEM and X-ray diffraction measurements (Figures 1 and 2). The Fe3O4-CV particles were monodisperse, with an average diameter of 9.4 ± 1.0 nm as determined from TEM images (results not shown here). The Fe3O4-CV powder was polycrystalline with diffraction peaks at: 30.368°, 35.748°, 43.368°, 53.718°, 57.218° and 62.778° which correspond to (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) reflection planes of fcc magnetite [42]. The peaks of Fe3O4-CV indexed in Figure 2 match with the standard pattern of Fe3O4 (ICDD card No. 19-0629).
The chemical composition and surface coverage of Fe3O4@CV films on G-tubes were evaluated by IR–mapping and SEM imaging (Figures 3–7). FT-IR spectral analysis of modified surfaces is very complicated because the absorption peaks often overlap with each other. This inconvenience is eliminated by using second derivative spectroscopy, a technique which enhances the separation of overlapping peaks [43]. For this purpose, second derivative IR-maps were recorded (an average of 250 IR spectra) on Fe3O4@CV films obtained in various conditions of MAPLE deposition, namely, at 300 mJ/cm2 , 400 mJ/cm2 and 500 mJ/cm2 laser fluences (Figures 3–5).
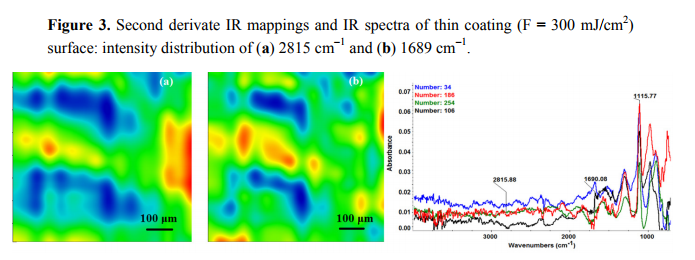
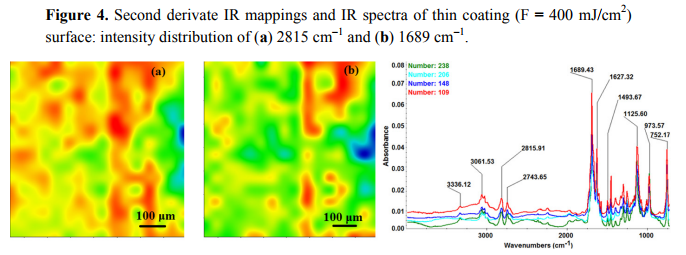
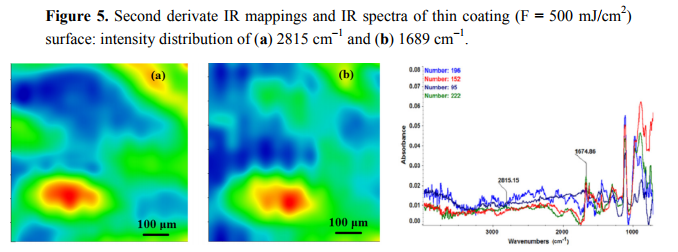
In order to verify if the MAPLE deposited films were chemically intact, the obtained IR maps were compared to drop-casted Fe3O4@CV films on G-tubes (Figure 6). The IR maps show colour changes, starting from blue (the lowest intensity) and gradually increasing to red (the highest intensity) which illustrate a high degree of film purity and chemical stability [44].
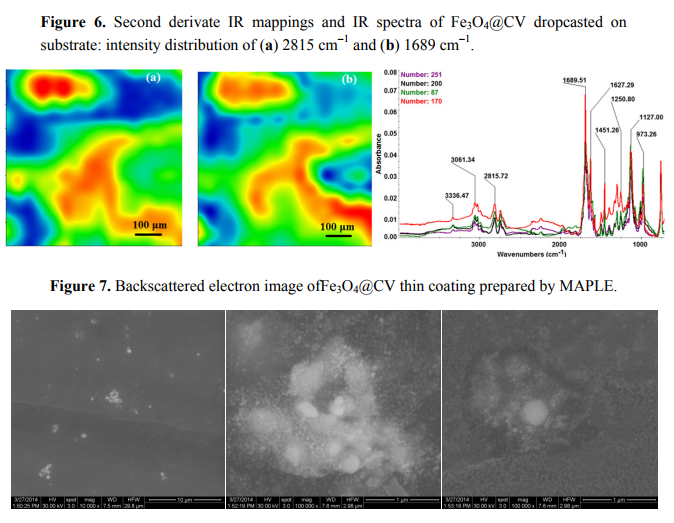
As it can be observed and compared in Figures 3–6, the optimal deposition rate and stoichiometric transfer was achieved at a fluence deposition of 400 mJ/cm2, the IR spectra of this MAPLE deposition showing similar features as the ones obtained for the drop-casted Fe3O4@CV films.
The characteristic peaks of the Fe3O4@CV were assigned to: (a) 2815 cm−1 (C-H stretch) and (b) 1689 cm−1 (C=O carbonyl group). The selected functional groups are characteristics to the C. verum (i.e., cinnamaldehyde). The surface coverage of Fe3O4@CV film on G-tubes deposited at 400 mJ/cm2 laser fluence and its topography were analysed by scanning electron microscopy (Figure 7). Both, very uniform surface coverage with Fe3O4@CV nanoparticles being well dispersed together with several aggregates were observed on the modified G-tubes (Figure 7). These results are in good agreement with reported literature on the MAPLE thin coatings of iron oxide nanoparticles [45].
Cytotoxicity assays revealed an improvement of the modified Fe3O4@CV G-tube surface biocompatibility with respect to the bare G-tube.
The MTT assay demonstrated that human cells present a normal metabolism and growth in the presence of nanoparticle modified bioactive G-tubes, the measured values of absorbance at 570 nm being similar for modified and control surfaces in the first 48 h of incubation. Furthermore, after 72 h of incubation, endothelial cells showed a better proliferation on Fe3O4@CV G-tube surfaces, as compared with the control surfaces (Figure 8).
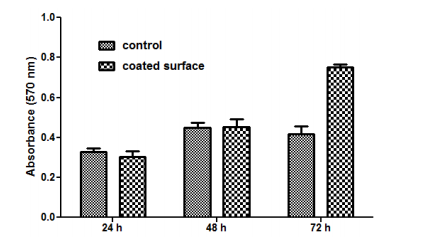
Florescence microscopy results obtained after 5 days of incubation have revealed that endothelial cells grown on the nanoparticle modified bioactive surfaces present a normal aspect, their morphology being similar with the cells grown on control surfaces (Figure 9).
The percentage of attached cells was normal for this incubation time and low detachment rates were noted. Our data revealed that the nanoparticle modified G-tube surfaces have improved anti-adherent properties, considerably reducing both Gram negative and Gram positive bacteria colonization. The phenotypical data demonstrate that biofilms formation is impaired at all tested time points, starting with their initiation and continuing through the maturation process.
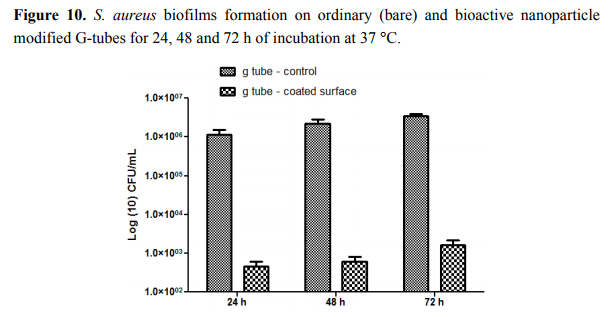
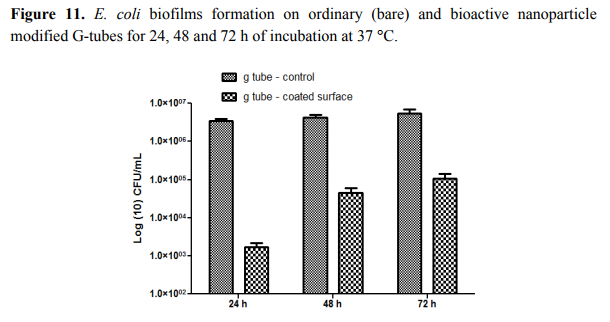
The most significant differences were observed on S. aureus biofilms; in this case, the biofilm inhibition rates ranging from more than 4-fold for incipient biofilms to up to 3-fold for mature biofilms (Figure 10). On the other hand, for the Gram negative bacterium E. coli the biofilm inhibition ranged from 2.5-fold for initial biofilms to up to 2-fold for mature biofilms grown onto the bioactive Fe3O4@CVmodified G-tube surface (Figure 11).
3. Experimental
3.1. Synthesis of Fe3O4@CV
Fe3O4 nanoparticles functionalized with C. verum were prepared as follow: NH4OH solution (25%, 8 mL) was added into a 0.25% solution of C. verum (200 mL). Then, a solution of Fe3+ and Fe2+ in 2:1 molar ratio was prepared (400 mL), mixed for 10 min and consecutively added dropwise to the basic solution of C. verum. A black precipitate was obtained consisting of C. verum functionalized magnetite nanoparticles (Fe3O4@CV) which were isolated from solution using a 100 kgf Nd-Fe-B external magnetic field. The Fe3O4@CV nanoparticles were purified by washing three times with Millipore water until the pH was near neutral. 3.2. MAPLE Target Preparation and Deposition of Fe3O4@CV-Based Thin Coating Fe3O4@CV nanoparticles were dispersed in DMSO as a 1.5% (w/v) solution. The MAPLE solutions were poured into a pre-cooled target holder and subsequently immersed in liquid nitrogen for 30 min. MAPLE depositions was performed using a KrF* (λ = 248 nm and τFWHM = 25 ns) COMPexPro 205 model laser source (Lambda Physics-Coherent, Ft. Lauderdale, FL, USA) that operated at a repetition rate of 15 Hz. The laser fluence was within the range of (300–500) mJ/cm2 whereas the laser spot area was set to 36 mm2
. A laser beam homogenizer was used to improve the energy distribution of the laser spot. In order to avoid the target heating and drilling during the laser irradiation, the frozen target was rotated at a rate of 0.4 Hz. All depositions were conducted at room temperature into a background pressure of 1 Pa and the films were grown at a target-substrate eparation distance of 5 cm by applying (30,000–60,000) subsequent laser pulses. During the deposition process, the target was kept at a temperature of ~173 K by active liquid nitrogen cooling. Thin films were deposited onto double side polished (100) silicon, glass and gastrostomy tubes (obtained from Doctor Anghel Medical Center) for IRM, SEM, XRD and biological assays, respectively. Prior to introduction inside the deposition chamber, the substrates were successively cleaned into an
ultrasonic bath with acetone, ethanol and deionized water for 15 min. During the deposition, the substrates were continuously rotated. Thus, the Fe3O4@CV nanoparticles were uniformly spread over the surface of the substrates. For data comparison, a control set of films were prepared by drop casting on the double side polished (100) silicon.
3.3. Characterization of Fe3O4@CV and Prepared Thin Coating
3.3.1. XRD
X-ray diffraction analysis was performed on a Shimadzu XRD 6000 diffractometer (Kyoto, Japan) at room temperature. In all the cases, Cu Kα radiation from a Cu X-ray tube (run at 15 mA and 30 kV) was used. The samples were scanned in the Bragg angle 2θ range of 10–80 degree.
3.3.2. IRM
IR mapping were recorded on a Nicolet iN10 MX FT-IR Microscope (Waltham, MA, USA) with MCT liquid nitrogen cooled detector in the measurement range 4000–700 cm−1 Spectral collection was made in reflection mode at 4 cm−1 resolution. For each spectrum, 32 scans were co-added and converted to absorbance using OmincPicta software (Thermo Scientific, Waltham, MA USA). Approximately 250 spectra were analysed for each sample. Two absorptions peaks known as being characteristics for the Fe3O4@CV nanoparticles were selected as spectral markers.
3.3.3. SEM
SEM analysis was performed on a FEI electron microscope (Hillsboro, OR, USA), using secondary electron beams with energies of 30 keV, on samples covered with a thin silver layer.
3.3.4. Interaction with Eukaryotic Cells Human endothelial cells (EAhy926 cell line, American Type Culture Collection ‒ ATCC, Manassas, VA, USA) were grown in DMEM culture medium containing 10% FBS, and 1% penicillin and neomycin (Sigma Aldrich, St. Louis, MO, USA). For cell proliferation and viability was used CellTiter96 Non-Radioactive Cell Proliferation Assay, (Promega, Madison, WI, USA). Endothelial cells were seeded in 96-well plate at a density of 5 × 103 cells/well, in DMEM medium, supplemented with 10% FBS and incubated with NMS materials for 72 h, while the controls were represented by endothelial cells grown in the same culture conditions, but on bare substrates. Cell proliferation assay was performed in triplicates, according to manufacturer’s guidelines, at different time intervals. Briefly, Promega Kit Solution I (15 µL) was added in each well and incubated for 4 h. Furthermore, Promega Kit Solution II (100 µL) was added in the 96-well plate and incubated for another hour and spectrophotometry measurements were performed at 570 nm using a Mithras LB 940 spectrophotometer (Berthold Technology, Bad Wildbad, Germany). RED CMTPX fluorophore (Life Technologies, Invitrogen, Antisel, Bucharest, Romania) is a cell tracker for long-term tracing of living cells. The RED CMTPX dye was added in the culture medium at a final concentration of 5 µM, incubated 30 min for the dye to penetrate through the cells. Furthermore, the cells were washed with PBS and visualized by fluorescent microscopy. Living cells tracing in the presence of thin coating was monitored for 5 days in culture. The micrographs were taken by a digital camera driven by the Axio-Vision 4.6 (Carl Zeiss, Oberkochen, Germany) software.
3.3.5. Interaction with Prokaryotic Cells
Staphylococcus aureus ATCC 25922 and Escherichia coli ATCC 25922 strains were purchased from the ATCC and used to create an artificial biofilm. Both adherence and biofilm formation were analysed in 6 multi-well plates (Nunc, Waltham, MA, USA), using a static model for monospecific biofilms. The tested slides pieces (control and MAPLE coated glass slides) were placed in 2 mL nutrient broth inoculated with 5 µL of S. aureus or E. coli suspension with a density of 0.5 McFarland (1–3 × 108 CFU/mL). After 24 h of incubation, the adherence was assessed by harvesting the adhered cells in sterile phosphate buffered saline (PBS), following the viable cell count assay. The temporal
dynamic of biofilm formation was studied by placing the tested slides with attached bacterial cells in nutrient broth and incubation for 24, 48 and 72 h. After incubation, the biofilm developed on the control and test surfaces were harvested and suspensions were performed in sterile PBS (phosphate aline buffer). The obtained suspensions were further ten-fold diluted and 10 µL of each dilution were seeded in triplicates on nutritive agar (Durham, NC, USA), incubated for 24 h at 37 °C in order to determine the viable cell counts and thus, indirectly, to evaluate the number of viable bacterial cells embedded in biofilms. Experiments were performed in triplicate and repeated on three separate occasions.
4. Conclusions
Thin films composed of 9.4 nm Cinnamomum verum-functionalized Fe3O4 particles were successfully transferred onto G-tubes by laser processing. The in vitro results recommend this type of coating for medical use, especially as a medical surface with high resistance to microbial colonization. The good biocompatibility of the Fe3O4-CV films suggests that they can be safely used for the antibacterial protection of medical surfaces and devices to be used for different patients with debilitating diseases.
Acknowledgments
The work has been supported by the Sectoral Operational Programme Human ResourcesDevelopment 2007-2013 of the Ministry of European Funds through the Financial Agreement POSDRU/159/1.5/S/132397 (ExcelDOC).
Author Contributions
AMG and IA conceived the study; AMH and AGA provided the microbial strains, and drafted the manuscript together with MC, GS and AMG. AMG, VG, GS, AEO and MC performed the synthesis, laser processing and characterization of Fe3O4@CV. AGA, AMH and FI performed the in vitro experiments. AMG, MC and IA participated in the design of the study and coordination. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
1. Locher, J.L.; Bonner, J.A.; Carroll, W.R.; Caudell, J.J.; Keith, J.N.; Kilgore, M.L.; Ritchie, C.S.; Roth, D.L.; Tajeu, G.S.; Allison, J.J. Prophylactic percutaneous endoscopic gastrostomy tube placement in treatment of head and neck cancer: A comprehensive review and call for evidence-based medicine. J. Parenter. Enter. Nutr. 2011, 35, 365–374.
2. Keung, E.Z.; Liu, X.; Nuzhad, A.; Rabinowits, G.; Patel, V. In-hospital and long-term outcomes after percutaneous endoscopic gastrostomy in patients with malignancy. J. Am. Coll. Surg. 2012, 215, 777–786.
3. Blomberg, J.; Lagergren, J.; Martin, L.; Mattsson, F.; Lagergren, P. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand. J. Gastroenterol. 2012, 47, 737–742.
4. Beech, I.B.; Sunner, J.A.; Arciola, C.R.; Cristiani, P. Microbially-influenced corrosion: Damage to prostheses, delight for bacteria. Int. J. Artif. Org. 2006, 29, 443–452.
5. Lazar, V. Quorum sensing in biofilms: How to destroy the bacterial citadels or their cohesion power? Anaerobe 2011, 17, 280–285.
6. Vickery, K.; Hu, H.; Jacombs, A.S.; Bradshaw, D.A.; Deva, A.K. A review of bacterial biofilms and their role in device-associated infection. Healthc. Infect. 2013, 18, 61–66.
7. Holban, A.M.; Cartelle Gestal, M.; Grumezescu, A.M. New molecular strategies for reducing implantable medical devices associated infections. Curr. Med. Chem. 2014, doi:10.2174/0929867321666140304103810.
8. Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123.
9. Tran, N.; Tran, P.A. Nanomaterial-Based treatments for medical device-associated infections. ChemPhysChem 2012, 13, 2481–2494.
10. Grumezescu, A.M. Improved wound dressings: Novel approaches. Int. J. Pharm. 2014, 463, 117–118.
11. Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Gheorghe, I.; Vlad, M.; Anghel, G.A.; Balaure, P.C.; Chifiriuc, C.M.; Ciuca, I.M. Improved activity of aminoglycosides entrapped in silica networks against microbial strains isolated from otolaryngological infections. Farmacia 2014, 62, 69–78.
12. Grumezescu, A.M.; Andronescu, E.; Voicu, G.; Huang, K.-S.; Yang, C.-H.; Ficai, A.; Vasile, B.S.; Grumezescu, V.; Bleotu, C.; Chifiriuc, M.C. New silica nanostructure for the improved delivery of topical antibiotics used in the treatment of staphylococcal cutaneous infections. Int. J. Pharm.
2013, 476, 170–176.
13. Anghel, I.; Grumezescu, A.M. Hybrid nano-structured coating for increased resistance of prosthetic devices to staphylococcal colonization. Res. Lett. 2013, 8, doi:10.1186/1556-276X-8-6.
14. Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Lazar, V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilms development. Nanoscale Res. Lett. 2012, 7, 690.
15. Colonna, C.; Dorati, R.; Conti, B.; Caliceti, P.; Genta, I. Sub-unit vaccine against S. aureus-mediated infections: Set-up of nano-sized polymeric adjuvant. Int. J. Pharm. 2013, 452, 390–401.
16. Nafee, N.; Youssef, A.; El-Gowelli, H.; Asem, H.; Kandil, S. Antibiotic-free nanotherapeutics: Hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int. J. Pharm. 2013, 454, 249–258.
17. Tan, S.P.; McLoughlin, P.; O’Sullivan, L.; Prieto, M.L.; Gardiner, G.E.; Lawlor, P.G.; Hughes, H. Development of a novel antimicrobial seaweed extract-based hydrogel wound dressing. Int. J. Pharm. 2013, 456, 10–20.
18. Belcarz, A.; Zima, A.; Ginalska, G. Biphasic mode of antibacterial action of aminoglycoside antibiotics-loaded elastic hydroxyapatite–glucan composite. Int. J. Pharm. 2013, 454, 285–295.
19. Echezarreta-López, M.M.; Landin, M. Using machine learning for improving knowledge on antibacterial effect of bioactive glass. Int. J. Pharm. 2013, 453, 641–647.
20. Grumezescu, A.M. Novel strategies to eradicate bacterial communities based on nano and biomaterials. Curr. Org. Chem. 2014, 18, 151.
21. Anghel, I.; Grumezescu, V.; Andronescu, E.; Anghel, G.A.; Grumezescu, A.M.; Mihaiescu, D.E.; Chifiriuc, M.C. Protective effect of magnetite nanoparticle/Salvia officinalis essential oil hybrid nanobiosystem against fungal colonization on the Provox® voice section prosthesis. Dig. J.
Nanomater. Bios. 2012, 7, 1205–1212.
22. Grumezescu, A.M.; Saviuc, C.; Chifiriuc, C.M.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; tanciu, G.; Lazar, V. Inhibitory activity of Fe3O4/Oleic acid/usnic acid—Core/shell/extra-shell nanofluid on S. aureus biofilm development. IEEE Trans. NanoBiosci. 2011, 10, 269–274.
23. Grumezescu, A.M.; Andronescu, E.; Albu, M.G.; Ficai, A.; Dragu, D. Wound dressing based collagen biomaterials containing usnic acid as quorum sensing inhibitor agent: Synthesis, characterization and bioevaluation. Curr. Org. Chem. 2013, 17, 125–131.
24. Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; VasileB. S.; Chifiriuc, M.C. In vitro activity of the new water dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15, 1766.
25. Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, C.M.; Radulescu, R. Magnetite-usnic acid nanostructured bioactive material with antimicrobial activity. Rom. J. Mater. 2013, 43, 402–407.
26. Mihaiescu, D.E.; Grumezescu, A.M.; Buteica, A.S.; Mogosanu, D.E.; Balaure, P.C.; Mihaiescu, O.M.; Trăistaru, V.; Vasile, B.S. Bioassay and electrochemical evaluation of controlled release behavior of cephalosporins from magnetic nanoparticles. Dig. J. Nanomater. Bios. 2012, 7, 253–260.
27. Grumezescu, V.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Ficai, A.; Vasile, B.S.; Trusca, R.; Bleotu, C.; Lazar, V.; Chifiriuc, C.M.; et al. Usnic acid loaded biocompatible magnetic PLGA-PVA microspheres thin films fabricated by MAPLE with increased resistance to staphylococcal
colonization. Biofabrication 2014, 6, 035002.
28. Saviuc, C.M.; Grumezescu, A.M.; Bleotu, C.; Holban, A.M.; Chifiriuc, M.C.; Balaure, P.; Lazar, V. Culture methods versus flow cytometry for the comparative assessment of antifungal activity of Eugenia caryophyllata thunb. (Myrtaceae) essential oil. Farmacia 2013, 61, 912–919.
29. Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Voicu, G.; Cocos, O.; Chifiriuc, M.C. Eugenia cayophyllata essential oil-SiO2 biohybrid structure for the potentiation of antibiotics activity. om. J. Mater. 2013, 43, 160–166.
30. Saviuc, C.; Cotar, A.I.; Holban, A.M.; Banu, O.; Grumezescu, A.M.; Chifiriuc, M.C. Phenotypic and molecular evaluation of Pseudomonas aeruginosa and Staphylococcus aureusvirulence patterns in the presence of some essential oils and their major compounds. Lett. Appl. NanoBiosci 2013, 2, 91–96.
31. Saviuc, C.; Holban, A.M.; Grumezescu, A.M.; Bleotu, C.; Banu, O.; Lazar, V.; Mihaiescu, D.E.; hifiriuc, M.C. Testing antifungal activity of some essential oils using flow cytometry. Lett. Appl. NanoBiosci. 2012, 1, 67–71.
32. Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343.
33. Song, J.; Bi, H.; Xie, X.; Guo, J.; Wang, X.; Liu, D. Natural borneol enhances geniposide ophthalmic absorption in rabbits. Int. J. Pharm. 2013, 445, 163–170.
34. Saviuc, C.; Grumezescu, A.M.; Banu, O.; Chifiriuc, C.; Mihaiescu, D.; Balaure, P.; Lazar, V. Biocompatible magnetic MWCNTs based on phytocomponents from Eugenia carryophyllata. ev. Chim. Bucharest 2012, 63, 531–535.
35. Saviuc, C.; Grumezescu, A.M.; Chifiriuc, M.C.; Mihaiescu, D.E.; Hristu, R.; Stanciu, G.; Oprea, E.; Radulescu, V.; Lazar, V. Hybrid Nanosystem for Stabilizing Essential Oils in Biomedical Applications. Dig. J. Nanomater. Bios. 2011, 6, 1657–1666.
36. Anghel, I.; Holban, A.M.; Andronescu, E.; Grumezescu, A.M.; Chifiriuc, M.C. Efficient surface functionalization of wound dressings byaphytoactive nanocoating refractory to Candida albicans biofilm development. Biointerphases 2013, 8, doi:10.1186/1559-4106-8-12.
24. Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; VasileB. S.; Chifiriuc, M.C. In vitro activity of the new water dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15, 1766.
25. Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Grumezescu, V.; Chifiriuc, C.M.; Radulescu, R. Magnetite-usnic acid nanostructured bioactive material with antimicrobial activity. Rom. J. Mater. 2013, 43, 402–407.
26. Mihaiescu, D.E.; Grumezescu, A.M.; Buteica, A.S.; Mogosanu, D.E.; Balaure, P.C.; Mihaiescu, O.M.; Trăistaru, V.; Vasile, B.S. Bioassay and electrochemical evaluation of controlled release behavior of cephalosporins from magnetic nanoparticles. Dig. J. Nanomater. Bios. 2012, 7, 253–260.
27. Grumezescu, V.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Ficai, A.; Vasile, B.S.; Trusca, R.; Bleotu, C.; Lazar, V.; Chifiriuc, C.M.; et al. Usnic acid loaded biocompatible magnetic PLGA-PVA microspheres thin films fabricated by MAPLE with increased resistance to staphylococcal
colonization. Biofabrication 2014, 6, 035002.
28. Saviuc, C.M.; Grumezescu, A.M.; Bleotu, C.; Holban, A.M.; Chifiriuc, M.C.; Balaure, P.; Lazar, V. Culture methods versus flow cytometry for the comparative assessment of antifungal activity of ugenia caryophyllata thunb. (Myrtaceae) essential oil. Farmacia 2013, 61, 912–919.
29. Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Voicu, G.; Cocos, O.; Chifiriuc, M.C. Eugenia cayophyllata essential oil-SiO2 biohybrid structure for the potentiation of antibiotics activity. Rom. J. Mater. 2013, 43, 160–166.
30. Saviuc, C.; Cotar, A.I.; Holban, A.M.; Banu, O.; Grumezescu, A.M.; Chifiriuc, M.C. Phenotypic and molecular evaluation of Pseudomonas aeruginosa and Staphylococcus aureusvirulence patterns in the presence of some essential oils and their major compounds. Lett. Appl. NanoBiosci 2013, 2, 91–96.
31. Saviuc, C.; Holban, A.M.; Grumezescu, A.M.; Bleotu, C.; Banu, O.; Lazar, V.; Mihaiescu, D.E.; Chifiriuc, M.C. Testing antifungal activity of some essential oils using flow cytometry. Lett. Appl. NanoBiosci. 2012, 1, 67–71.
32. Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343.
33. Song, J.; Bi, H.; Xie, X.; Guo, J.; Wang, X.; Liu, D. Natural borneol enhances geniposide ophthalmic absorption in rabbits. Int. J. Pharm. 2013, 445, 163–170.
34. Saviuc, C.; Grumezescu, A.M.; Banu, O.; Chifiriuc, C.; Mihaiescu, D.; Balaure, P.; Lazar, V. Biocompatible magnetic MWCNTs based on phytocomponents from Eugenia carryophyllata. Rev. Chim. Bucharest 2012, 63, 531–535.
35. Saviuc, C.; Grumezescu, A.M.; Chifiriuc, M.C.; Mihaiescu, D.E.; Hristu, R.; Stanciu, G.; Oprea, E.; Radulescu, V.; Lazar, V. Hybrid Nanosystem for Stabilizing Essential Oils in Biomedical Applications. Dig. J. Nanomater. Bios. 2011, 6, 1657–1666.
36. Anghel, I.; Holban, A.M.; Andronescu, E.; Grumezescu, A.M.; Chifiriuc, M.C. Efficient surface functionalization of wound dressings by a phytoactive nanocoating refractory to Candida albicans biofilm development. Biointerphases 2013, 8, doi:10.1186/1559-4106-8-12.
